Get Healthy!
Staying informed is also a great way to stay healthy. Keep up-to-date with all the latest health news here.
17 Jul
Added Sugars and Artificial Sweeteners Linked to Early Puberty in Children
A new study finds both added sugars and artificial sweeteners are associated with a higher risk of early puberty, especially in children with a genetic predisposition.
16 Jul
PMS and Other Premenstrual Disorders Linked to Increased Risk of Heart Disease
Young women who suffer from PMS or PMDD are 10% more likely to develop heart disease later in life, a new study finds.
15 Jul
Nicotine Pouch Poisonings Soar in Young Children
Calls to U.S. poison control centers involving nicotine pouches and young kids soared 763% from 2020 to 2023.
Hispanic People Have Unexplained Higher Risk For Nerve Disorder
Hispanic people are more likely to develop peripheral neuropathy than white people, and it’s not clear why, a new study has found.
Hispanic folks were 32% more likely than white people to have this nerve disorder even after accounting for known health, lifestyle and social risk factors, researchers reported July 16 in the journal
- Dennis Thompson HealthDay Reporter
- |
- July 18, 2025
- |
- Full Page
Diabetic Women Should Be Asked About Desire For Kids At Every Doctor's Visit, Guidelines Say
Doctors should ask diabetic women at every visit about their intention to have a child, to make sure they get the appropriate care prior to conception, new guidelines say.
This will help avoid miscarriages and birth defects among women who have diabetes before pregnancy, the authors write in the Journal of Clinical Endocrinology & ...
- Dennis Thompson HealthDay Reporter
- |
- July 18, 2025
- |
- Full Page
Nieces, Nephews Become Dementia Caregivers Unexpectedly, Study Says
Two-thirds of nieces and nephews caring for an aging relative with dementia never expected to wind up with that responsibility, a recent small-scale study says.
These caregivers say they either gradually fell into the role, or had it forced upon them by family circumstances, according to findings published recently in the journal The G...
- Dennis Thompson HealthDay Reporter
- |
- July 18, 2025
- |
- Full Page
Depression Risk Greater In Some Women With Premature Menopause
Some women have a greater risk of depression as they go through premature menopause, according to a new study.
Premature menopause occurs when the ovaries stop functioning normally before age 40, researchers said in background notes.
The condition has been linked with a more than tripled risk for depression and nearly quintupled risk...
- Dennis Thompson HealthDay Reporter
- |
- July 18, 2025
- |
- Full Page
What Are The Best Treatments For Chronic Hives?
Hives can drive a person crazy, covering parts of the body with maddeningly itchy welts.
Now, a new evidence review has identified the most effective treatments for people who suffer from chronic hives.
The injectable antibody drug omalizumab and a yet-to-be-approved pill called remibrutinib are among the most effective treatments fo...
- Dennis Thompson HealthDay Reporter
- |
- July 18, 2025
- |
- Full Page
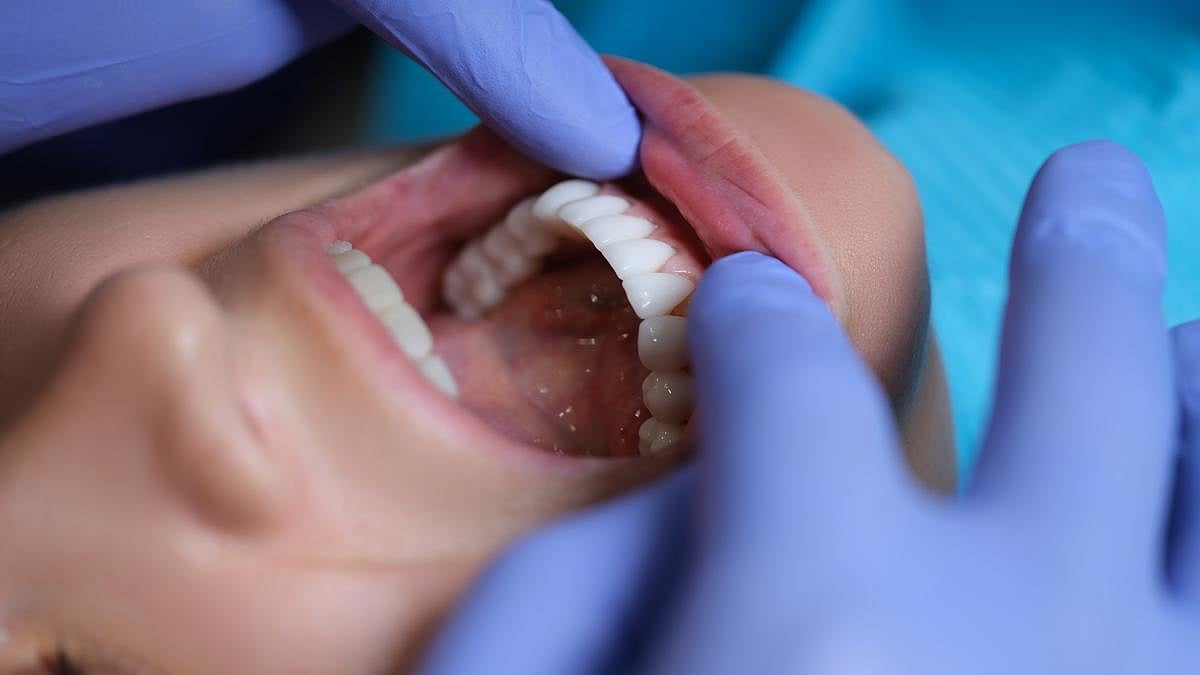
Poor Oral Health Potentially Linked To Chronic Health Problems
Poor dental health could be a harbinger of chronic illnesses like type 2 diabetes and heart disease, a new study warns.
People with missing teeth, coated tongues and other signs of poor oral health also were more likely to have elevated blood sugar, high cholesterol and diminished kidney function, researchers reported recently in the J...
- Dennis Thompson HealthDay Reporter
- |
- July 18, 2025
- |
- Full Page
Florida House Cat's Latest Conquest Yields New Virus Discovery
Florida virologist John Lednicky couldn’t have a more devoted research partner than his sleek black house cat Pepper.
Pepper, an avid hunter who often leaves "gifts" for his people, made news last year for his role in helping detect the arrival in the U.S. of an exotic germ called jeilongvirus. Now, he’s at it again.
Thi...
- Carole Tanzer Miller HealthDay Reporter
- |
- July 17, 2025
- |
- Full Page
Living Near Polluted Missouri Creek as a Child Tied to Later Cancer Risk
Folks who grew up near a polluted Missouri creek during the 1940s through 1960s may have higher odds for cancer now, new research shows.
The study focused on Coldwater Creek in St. Louis County. The area was contaminated with radioactive waste from the U.S. government’s atomic bomb program during World War II.
Back then, uraniu...
- I. Edwards HealthDay Reporter
- |
- July 17, 2025
- |
- Full Page
RFK Jr. Fires Two Trump-Appointed Senior Officials in Ongoing HHS Restructuring
U.S. Health and Human Services (HHS) Secretary Robert F. Kennedy Jr. has removed two senior officials who were appointed by President Donald Trump.
Heather Flick Melanson, chief of staff, and Hannah Anderson, a top policy adviser, were both let go recently. The decision came as a surprise to several federal health officials.
Both wom...
- I. Edwards HealthDay Reporter
- |
- July 17, 2025
- |
- Full Page
Playing A Musical Instrument Might Help Aging Brains
Want to help maintain your brain health as you age?
Then pick up a guitar, start tickling a piano’s ivories or join a band.
Playing an instrument can promote a youthful pattern of brain activity, researchers reported July 15 in the journal PLOS Biology.
Specifically, older musicians were better able to understand...
- Dennis Thompson HealthDay Reporter
- |
- July 17, 2025
- |
- Full Page
Insomniacs With Inflammation Prone To Depression
Insomniacs have a much higher risk for depression if they have chronic inflammation, a new sleep lab experiment says.
Seniors with insomnia were three times as likely to report symptoms of depression if they’d been dosed with a substance that promotes inflammation, according to results published July 16 in JAMA Psychiatry.
- Dennis Thompson HealthDay Reporter
- |
- July 17, 2025
- |
- Full Page
Some Newer Antiseizure Meds Safer During Pregnancy
Some newer antiseizure medications appear to be safer for pregnant women to take without risk of birth defects, a new study says.
Second-generation antiseizure drugs like levetiracetam, oxcarbazepine, gabapentin and zonisamide did not show an increased risk for birth defects, researchers reported July 16 in the journal Neurology.<...
- Dennis Thompson HealthDay Reporter
- |
- July 17, 2025
- |
- Full Page
Belly Fat Increases Stress Incontinence Risk
Middle-aged women with more belly fat have a higher risk for stress urinary incontinence, a recent study says.
Fat around the waist and visceral organs increases by 33% a woman’s risk of leaking when she sneezes, coughs or exerts herself, researchers reported in the journal Menopause.
“Abdominal obesity may cause...
- Dennis Thompson HealthDay Reporter
- |
- July 17, 2025
- |
- Full Page
Few Babies Getting RSV Antibody Shot, Study Says
A new antibody shot that protects babies against RSV infection could be struggling to gain traction, researchers report.
Only about a third (35%) of babies eligible for nirsevimab got the injection during the 2023-24 RSV season, researchers reported today in the journal Pediatrics.
That was the first season that the monoclon...
- Dennis Thompson HealthDay Reporter
- |
- July 17, 2025
- |
- Full Page
Chronically Ill Kids Carry Heavy Emotional Burden
Kids coping with chronic health problems like asthma also are struggling with the emotional burden of stress, fear and sadness, a new study says.
Almost 94% of comments posted online by chronically ill kids and their caregivers expressed negative feelings like disgust, sadness and fear, according to results published recently in the Jo...
- Dennis Thompson HealthDay Reporter
- |
- July 17, 2025
- |
- Full Page
Thousands Laid off From NIH, FDA and CDC After Supreme Court Decision
Thousands of health workers lost their jobs this week after a U.S. Supreme Court ruling cleared the way for the Trump administration to move forward with major staffing cuts.
On Monday, the U.S. Department of Health and Human Services (HHS) finalized 10,000 layoffs across federal health agencies, including the National Institutes of Health...
- I. Edwards HealthDay Reporter
- |
- July 16, 2025
- |
- Full Page
Popular YoCrunch Yogurt Recalled Over Plastic Pieces in Packaging
YoCrunch yogurt products are being pulled from store shelves nationwide due to a safety concern, its manufacturer announced Monday.
Danone U.S., the maker of YoCrunch, said small, sharp pieces of plastic may be inside the dome toppers of some products, CNN reported.
The plastic could cause choking or injury if eaten. T...
- I. Edwards HealthDay Reporter
- |
- July 16, 2025
- |
- Full Page
Court Allows West Virginia to Restrict Abortion Pill Mifepristone
A federal appeals court has ruled that West Virginia can limit access to mifepristone, a medication used to end early pregnancies.
The decision is the first of its kind and could affect how other states handle access to drugs that are approved by the U.S. Food and Drug Administration (FDA).
Mifepristone is part of a two-drug regimen ...
- I. Edwards HealthDay Reporter
- |
- July 16, 2025
- |
- Full Page
Loneliness Of Widowhood Isn't Diminished By Presence Of Adult Children, Study Says
Adult children aren’t likely to fill the void left by the loss of a spouse, a new study says.
Becoming widowed might cause a stronger bond between the remaining parent and their children, but these bonds don’t appear to ease the loneliness left by loss, researchers reported July 14 in Aging & Mental Health.
T...
- Dennis Thompson HealthDay Reporter
- |
- July 16, 2025
- |
- Full Page
AI Can Help Screen For Vision-Destroying Diabetic Eye Disease
A new AI-powered retina tracker can help doctors screen for a vision-destroying diabetic eye disease, researchers say.
The Simple Mobile AI Retina Tracker (SMART) program achieved greater than 99% accuracy in screening for diabetic retinopathy, researchers reported Monday at the annual meeting of The Endocrine Society in San Francisco.
...- Dennis Thompson HealthDay Reporter
- |
- July 16, 2025
- |
- Full Page